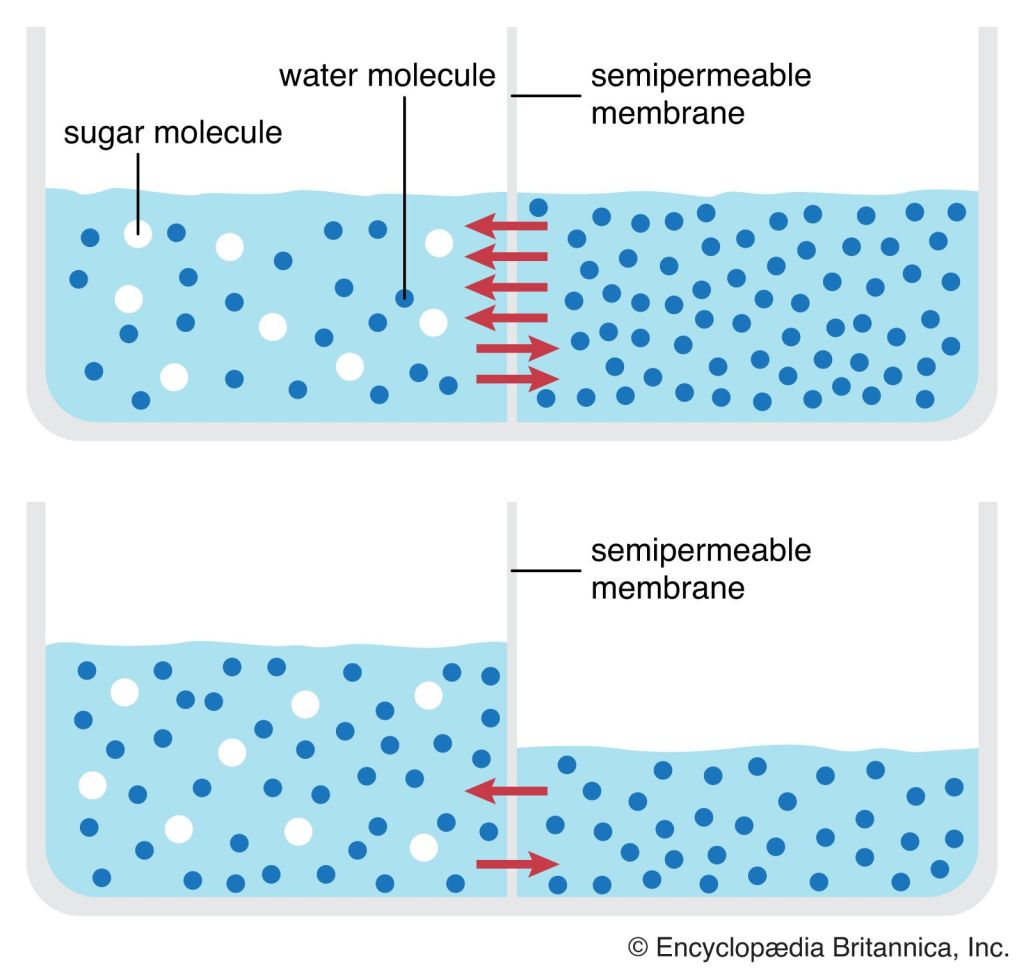
Osmosis is a biological process that involves the movement of solvent molecules (usually water) through a semipermeable membrane from an area of lower solute concentration to an area of higher solute concentration. It is a form of passive transport, meaning it does not require energy input from the cell.
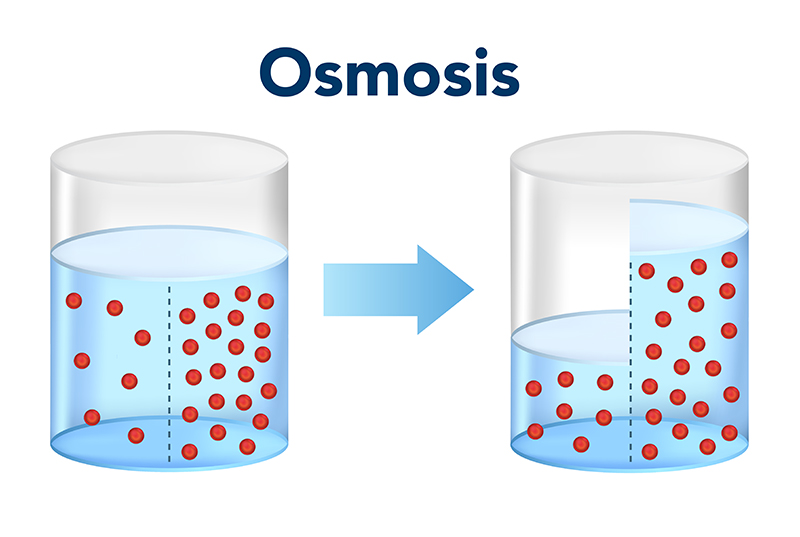
The semipermeable membrane allows the passage of solvent molecules but restricts the movement of solute molecules based on their size and charge. The direction of osmosis is determined by the relative concentrations of solute molecules on either side of the membrane. Water molecules will move across the membrane to equalize the concentration of solute particles on both sides.
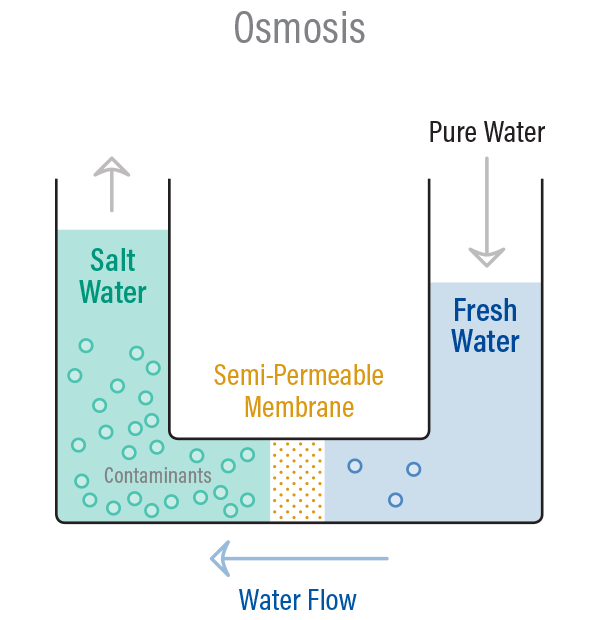
If the concentration of solute is higher outside the cell (hypertonic solution), water will move out of the cell through osmosis, causing the cell to shrink or become dehydrated. Conversely, if the concentration of solute is higher inside the cell (hypotonic solution), water will move into the cell through osmosis, causing the cell to swell or even burst. When the concentrations are equal on both sides (isotonic solution), there is no net movement of water.
Osmosis plays a crucial role in various biological processes, such as the absorption of water by plant roots, the movement of water in the human body, and the regulation of cell volume. It is also utilized in various practical applications, including water purification, dialysis, and preservation techniques like osmotic dehydration and osmotic drying.